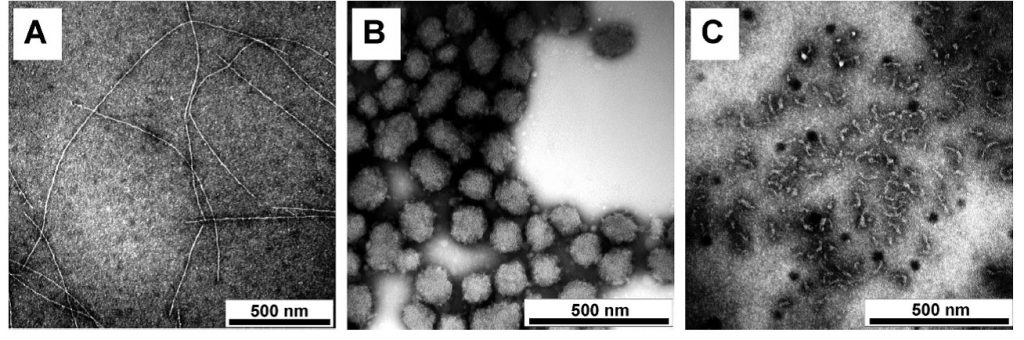
Aggregates with three different morphologies occur depending on the conditions [1]. Aggregates consisting of randomly connected relatively thin strands result from strong electrostatic repulsion. The size of these fractal aggregates at steady state increases with the protein concentration until, at a critical gel concentration, the aggregates percolate and form a system-spanning network. At a given concentration, larger aggregates emerge if the electrostatic repulsion between proteins is weaker, either because the net charge density is lower or because salt has been added.
We have shown that relatively dense roughly spherical microgels with radii between 50 and 200 nm occur if the net charge density of the proteins is below a critical value either close to pI or in the presence of divalent cations. Under most conditions, the microgels randomly stick together and either precipitate or, at higher concentrations, form a gel. However, within narrow pH ranges around pI or a narrow range of Ca2+ concentrations, stable suspensions of microgels arise. Finally at pH ≤ 2.5, the proteins hydrolyze into smaller peptides, which subsequently assemble into rigid fibrils.
In recent years, we have investigated the viscosifying and gelling properties of protein aggregates. Large fractal protein aggregates have a low density and can be used as viscosifiers, as the viscosity of suspensions of such aggregates increases rapidly with increasing concentration [2]. On the other hand, microgels of the same size are dense and can therefore be used to increase the protein content of products without significantly increasing the viscosity. Contrary to native proteins, aggregates formed at a steady state are stable when heated and therefore resist pasteurization. However, the aggregates associate and may form a gel when the electrostatic repulsion is reduced either by bringing the pH closer to pI or by adding salt [3]. It can be induced at low temperatures contrary to native proteins though the rate of this process decreases with decreasing temperature. Therefore, it is sometimes termed cold gelation; this can also occur in suspensions of microgels, but for a given protein concentration, stiffer gels are formed with fractal aggregates.
Protein aggregates can also stabilize oil-in-water emulsions and foams [4]. Due to their particle character, aggregates provide greater stability than native globular proteins. This is particularly striking for water in water emulsions formed by mixing aqueous solutions of incompatible polymers that cannot be stabilized by native proteins, but we have found that it can be done with protein aggregates. [5].
References:
[1] J. M. Jung et al., Biomacromolecules 9, 2477 (2008).
[2] W. Inthavong et al., Soft Matter (2016).
[3] A. Kharlamova, T. Nicolai, and C. Chassenieux, Food Hydrocolloids (2017).
[4] E. Dickinson, Food Hydrocolloids 68, 219 (2017).
[5] A. Gonzalez-Jordan, L. Benyahia, and T. Nicolai, Langmuir (2016).