Water’s unique thermodynamic anomalies, manifesting even under Earth’s ambient conditions, hold the key to understanding fundamental mechanisms driving life and Earth’s processes. Finding evidence nowadays in several molecular dynamics simulation studies, a compelling hypothesis for explaining the existence of water’s equilibrium anomalies is the so-called liquid-liquid phase transition (LLPT) between high-density and low-density liquid phases (HDL/LDL) in a supercooled, metastable liquid state. Its origin has been so-far not clarified. On the other hand, water is a polar liquid and, as such, can, in principle, undergo a ferroelectric phase transition, resulting in the setting of macroscopic polarization, under proper thermodynamic conditions.
Capillary Waves Found Between Fully Miscible Fluids
15th December 2025
Capillary Waves Found Between Fully Miscible Fluids
15th December 2025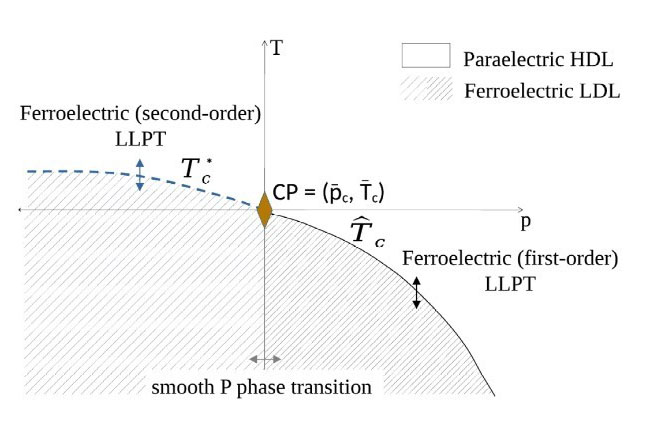
A recent study lead by a researcher from SoftComp partner Ca’ Foscari University of Venice unveiled not only a link between ferroelectric and liquid-liquid phase transitions, but also a possible role of ferroelectricity in promoting the LLPT, by analysing extensive molecular dynamics simulations and developing a classical density functional theory. The theory treats water as a polar liquid. Grounded in the characteristics of the microscopic dipolar potential interaction and the liquid’s molecules annealed positional disorder, it leads to a free energy expression that supports phase transitions both ferroelectric and liquid-liquid. This research not only characterizes but can shed light on the origin of the LLPT and thermodynamic anomalies in water.
Significant questions arise
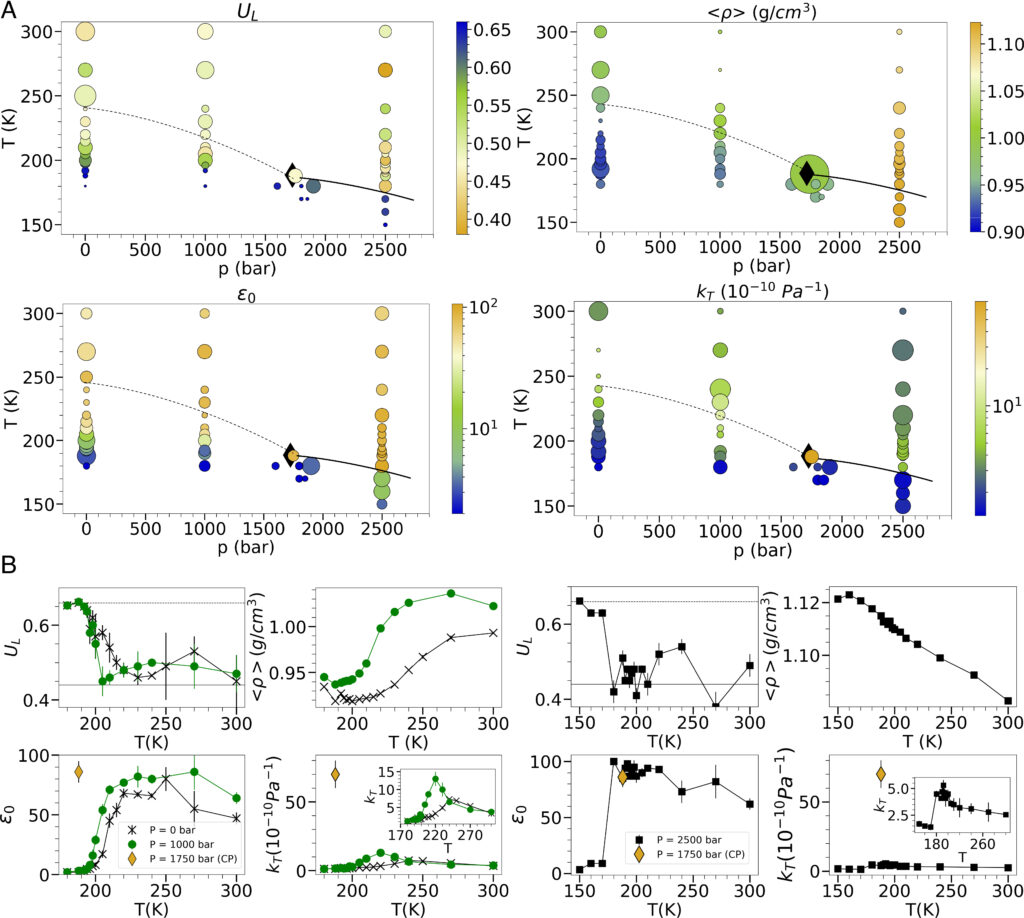
“By demonstrating that the overlooked dipolar degrees of freedom can actually play a leading role in the liquid-liquid phase transition in water, this research seeks to open thought-provoking perspectives”, says first author Maria Grazia Izzo. “Although the expression for free energy enabling phase transitions applies universally to polar liquids, its specific coefficients, which determine whether the transitions effectively occur under certain thermodynamic conditions, depend on the microscopic details. Our research thus raises significant questions: Is water ‘merely’ a polar liquid with the microscopic characteristics suitable to allow a ferroelectric phase transition close to Earth’s environmental conditions? And what contribute to shaping its microscopic characteristics?”
Read more: M.G. Izzo, J. Russo, G. Pastore, PNAS 121 (47) e2412456121 (2024) https://doi.org/10.1073/pnas.2412456121
SoftComp partners: Ca’ Foscari University of Venice
Images Copyright: 2024 the Authors. Published by PNAS under Creative Commons Attribution-NonCommercial License 4.0 (CC BY-NC-ND).
