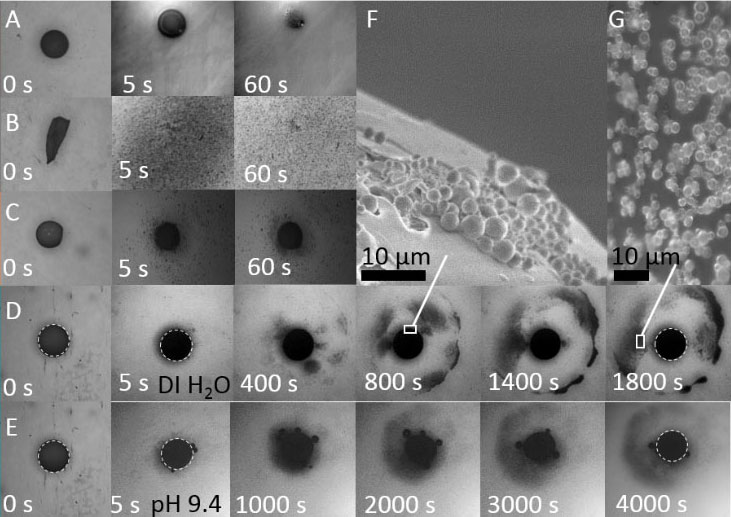
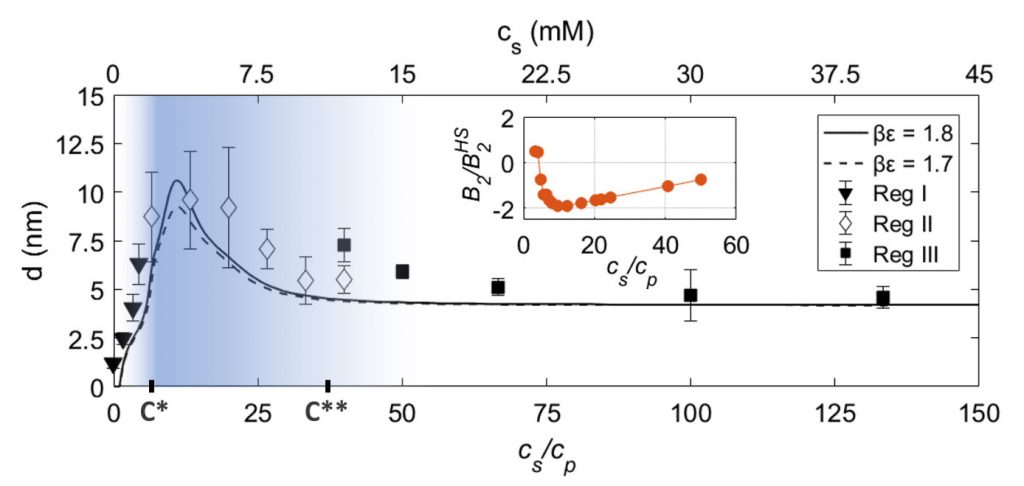
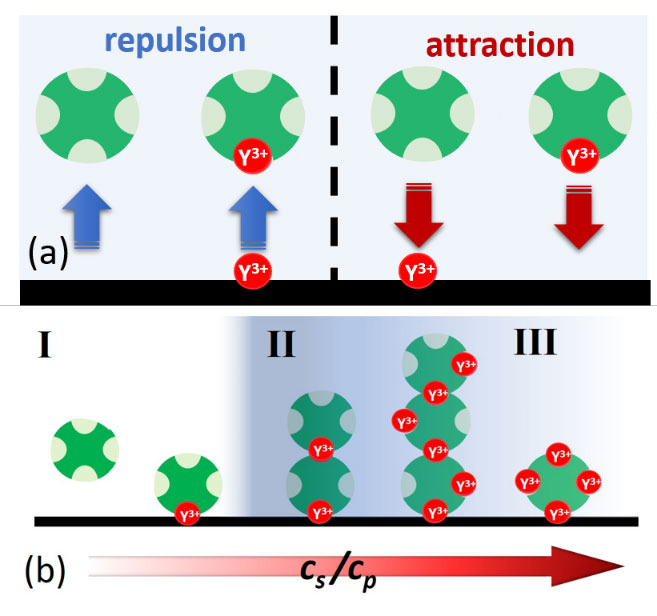
Protein adsorption at the solid-liquid interface is an important phenomenon that often can be observed as a first step in biological processes. Despite its inherent importance, still relatively little is known about the underlying microscopic mechanisms. Here, using multivalent ions, we demonstrate the control of the interactions and the corresponding adsorption of net-negatively charged proteins (bovine serum albumin) at a solid-liquid interface. This is demonstrated by ellipsometry and corroborated by neutron reflectivity and quartz-crystal microbalance experiments. We show that the reentrant condensation observed within the rich bulk phase behavior of the system featuring a nonmonotonic dependence of the second virial coefficient on salt concentration cs reflected in an intriguing way in the protein adsorption d(cs) at the interface. Our findings are successfully described and understood by a model of ion-activated patchy interactions within the framework of classical density functional theory. In addition to the general challenge of connecting bulk and interface behavior, our work is expected to have implications for, inter alia, nucleation at interfaces including protein crystallization.
Read more:
Fries M. R. et al.,
Phys. Rev. Lett. 2017; 119, 228001.
DOI: 10.1103/PhysRevLett.119.228001
SoftComp partner: University of Tuebingen, Germany