Impact of Aspect Ratio on Protein Network Assembly
Interconnected networks of high aspect ratio (AR) bio-polymers are ubiquitous in nature. A research team from SoftComp partner Leeds and Oxfordshire has now shown why high AR bio-polymer building blocks confer significant functional advantages. These include; increased mechanical strength at equivalent protein concentrations; and the rapid assembly of homogenous networks, above a critical concentration, crucial for in vivo biological processes e.g. blood clotting. In addition to uncovering the function advantages of filamentous proteins for hydrogel formation in vivo, manipulating AR also provides a novel parameter in the design of new biomaterials.
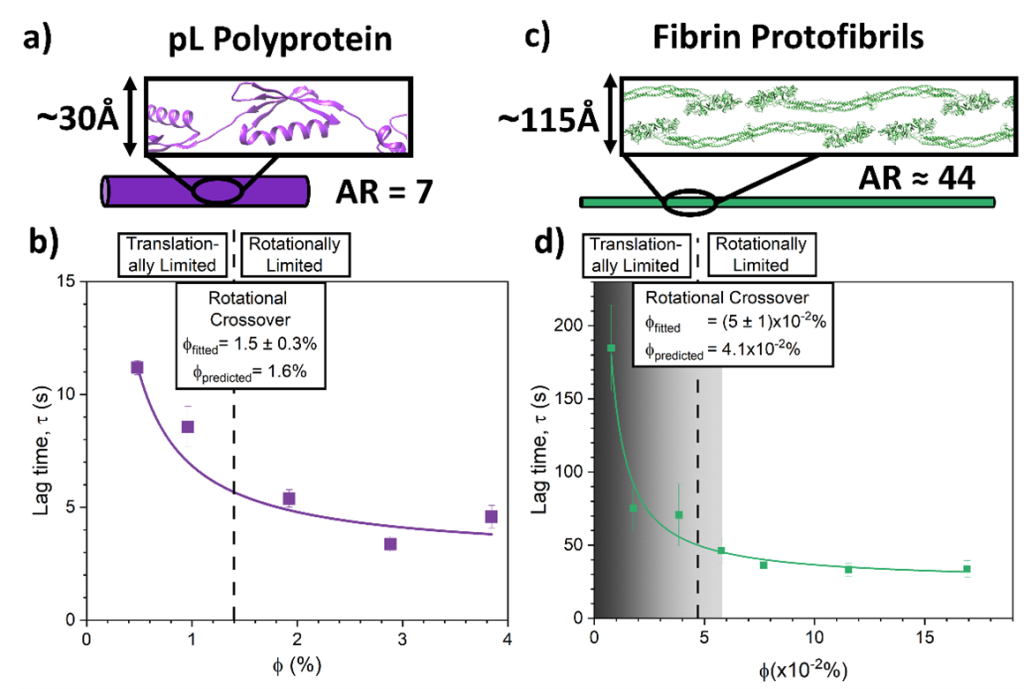
To shed light on the advantages of high aspect biopolymers as network building blocks, the scientists engineered proteinaceous building blocks with varying numbers of protein L (pL) domains, creating seven building blocks with ARs from one to seven. Using shear rheology and small-angle neutron scattering (SANS) to characterise the mechanical and structural properties of photochemically crosslinked pL networks at different volume fractions, φ, they showed that AR is a crucial property that defines network architecture and mechanics. Networks constructed from higher AR building blocks exhibit more homogeneous structures and higher storage moduli due to a shift from translational diffusion limited (TDL) to rotationally diffusion limited (RDL) network formation because building blocks with increased AR lack the space to rotate freely (Figure 1). The transition between these regimes is modelled by the geometric free rotation limit of a rigid cylindrical rod, also known as the transition from the dilute to the semi-dilute regime for rod-like particles.
Formation kinetics lag time, τ, measurements (supplemented with dynamic light scattering) of pL polyproteins, as a function of volume fraction, further demonstrate the presence of the TDL to RDL transition (Fig. 2a,b). Where the fitted crossover volume fraction, φcrit, extracted using a mean free path based empirical fit (Fig. 2 caption), matches the values determined by a geometric free rotation limit model (Eqn 1). Finally, for comparison, the scientists studied a fibrin network (Fig. 2c,d) and observe the same transition from TDL to RDL formation, confirming that living systems exploit AR for their network assembly.
Read more:
Hughes M. D. G. et al., Nat. Commun. 14, 5593 (2023)
SoftComp partner:
University of Leeds