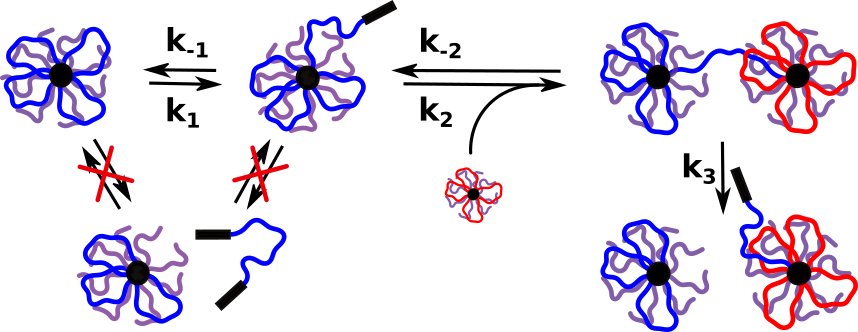
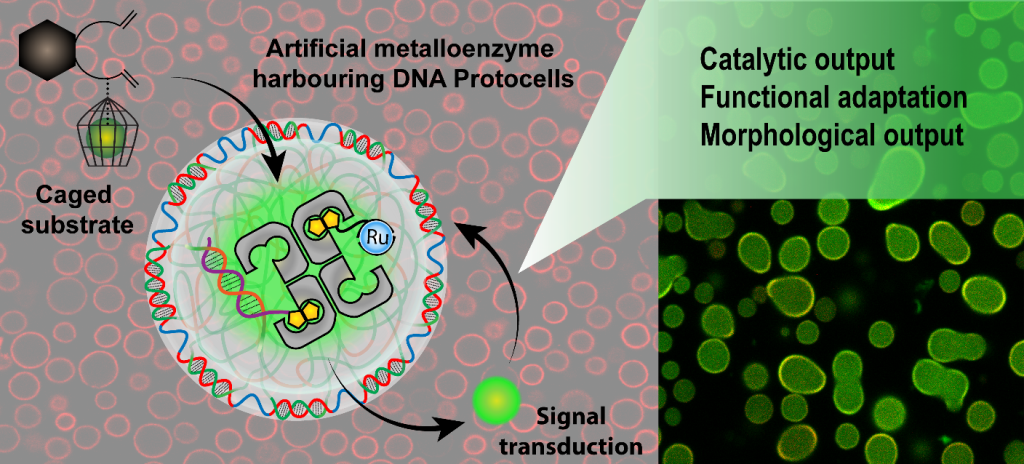
Protocells are self-organized, endogenously ordered, spherical systems usually formed by lipids, that are thought to be an important stage in the origin of life. The ability to mimic elementary aspects of cellular non-equilibrium processes in protocell models could also pave the way towards designing life-like systems orchestrating complex spatio-temporal transformations. Recently, we unravelled polymer-like phase-separation behaviour in multiblock single-stranded DNA by activating a nucleobase-specific lower critical solution temperature, which provides an opportunity to fabricate all-DNA protocells (PC) with several encoded sequences for post-functionalization with spatio-temporal control.
Furthermore, we introduced a functional and morphological adaptation concept driven by a bio-orthogonal metabolic reaction in these PCs. To achieve a metabolic transformation, we encapsulated a genetically modified artificial metalloenzyme (ArM) whose ring-closing metathesis activity on a pro-fluorescent substrate generated a green-fluorescent metabolite that weakened the A-T (adenine-thymine) duplexes of the PC shell by intercalation. This metabolic reaction led to PC growth, DNA mechanosensor activation, and interparticle PC fusion. Genetic engineering of the metalloenzyme increased the catalytic efficiency, and we observed significant molecular crowding effects.
Even though interdisciplinary techniques to explore the design, structure, function, and evolutionary potential of metabolic PCs with genetically evolved catalysts are in their infancy, our approach offers valuable insights into how chemically triggered adaptive behaviour of prebiotic coacervates can be achieved, and is a step towards the minimalistic design of life-like abiotic systems.
Read more:
Samanta, A. et al., Nat. Nanotechnol. 15, 914 (2020)
SoftComp partner:
Johannes Gutenberg University Mainz